Abstract
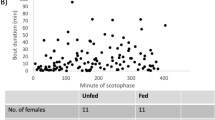
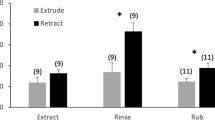
Female moths release sex pheromone to attract mates. In most species, sex pheromone is produced in, and released from, a specific gland. In a previous study, we used empirical data and compartmental modeling to account for the major pheromone gland processes of female Chloridea virescens: synthesis, storage, catabolism and release; we found that females released little (20–30%) of their pheromone, with most catabolized. The recent publication of a new pheromone collection method led us to reinvestigate pheromone release and catabolism in C. virescens on the basis that our original study might have underestimated release rate (thereby overestimating catabolism) due to methodology and females not calling (releasing) continuously. Further we wished to compare pheromone storage/catabolism between calling and non-calling females. First, we observed calling intermittency of females. Then, using decapitated females, we used the new collection method, along with compartmental modeling, gland sampling and stable isotope labeling, to determine differences in pheromone release, catabolism and storage between (forced) simulated calling and non-calling females. We found, (i) intact 1 d females call intermittently; (ii) pheromone is released at a higher rate than previously determined, with simulations estimating that continuously calling females release ca. 70% of their pheromone (only 30% catabolized); (iii) extension (calling)/retraction of the ovipositor is a highly effective “on/off’ mechanism for release; (iv) both calling and non-calling females store most pheromone on or near the gland surface, but calling females catabolize less pheromone; (v) females are capable of producing and releasing pheromone very rapidly. Thus, not only is the moth pheromone gland efficient, in terms of the proportion of pheromone released Vs. catabolized, but it is highly effective at shutting on/off a high flux of pheromone for release.






Similar content being viewed by others
References
Allison JD, Cardé RT (eds) (2016a) Pheromone communication in moths: evolution, behavior and application. University of Caifornia Press, Oakland
Allison JD, Cardé RT (2016b) Variation in moth pheromone: causes and consequences. In: Allison JD, Cardé RT (eds) Pheromone communication in moths: evolution, behavior and application. University of California Press, Oakland, pp 25–41
Almeida ÂA, Lima ER, Reis R Jr (2008) Pupal period affects calling behavior of the wheat moth, Pseudaletia sequax (Lepidoptera: Noctuidae). Ethology 114:499–503. https://doi.org/10.1111/j.1439-0310.2008.01492.x
Bendib A, Minet J (1998) Female pheromone glands in arctiidae (Lepidoptera). Evolution and phylogenetic significance Comptes Rendus de l’Académie des Sciences – Series III – Sciences de la Vie 321:1007–1014. https://doi.org/10.1016/S0764-4469(99)80056-0
Bjostad LB, Wolf WA, Roelofs WL (1987) Pheromone biosynthesis in lepidopterans: desaturation and chain shortening. In: Prestwich GD, Blomquist GJ (eds) Pheromone biochemistry. Academic Press, New York, pp 77–120
Chinkes DL, Aarsland A, Rosenblatt J, Wolfe RR (1996) Comparison of mass isotopomer dilution methods used to compute VLDL production in vivo. Am J Physiol Endocrinol Metab 271:E373–E383
Eltahlawy H, Buckner JS, Foster SP (2007) Evidence for two-step regulation of pheromone biosynthesis by the pheromone biosynthesis-activating neuropeptide in the moth Heliothis virescens. Arch Insect Biochem Physiol 64:120–130
Foster SP (1993) Neural inactivation of sex pheromone production in mated lightbrown apple moths, Epiphyas postvittana (Walker). J Insect Physiol 39:267–273. https://doi.org/10.1016/0022-1910(93)90098-C
Foster SP (2016) Toward a quantitative paradigm for sex pheromone production in moths. In: Allison JD, Cardé RT (eds) Pheromone communication in moths: evolution, behavior, and application. University of California Press, Oakland, pp 113–126
Foster S, Anderson K (2011) The use of mass isotopomer distribution analysis to quantify synthetic rates of sex pheromone in the moth Heliothis virescens. J Chem Ecol 37:1208–1210
Foster SP, Anderson KG (2018) Differential pheromone sampling of the gland of female Heliothis virescens moths reveals glandular differences in composition and quantity. J Chem Ecol 44:452–462. https://doi.org/10.1007/s10886-018-0954-0
Foster SP, Anderson KG (2019) Production and distribution of aldehyde and alcohol sex pheromone components in the pheromone gland of females of the moth Chloridea virescens. J Chem Ecol 45:9–17. https://doi.org/10.1007/s10886-018-1041-2
Foster SP, Anderson KG, Casas J (2017) Sex pheromone in the moth Heliothis virescens is produced as a mixture of two pools: de novo and via precursor storage in glycerolipids. Insect Biochem Mol Biol 87:26–34. https://doi.org/10.1016/j.ibmb.2017.06.004
Foster SP, Anderson KG, Casas J (2018) The dynamics of pheromone gland synthesis and release: a paradigm shift for understanding sex pheromone quantity in female moths. J Chem Ecol 44:525–533. https://doi.org/10.1007/s10886-018-0963-z
Groot AT (2014) Circadian rhythms of sexual activities in moths: a review. Front Ecol Evol 2. https://doi.org/10.3389/fevo.2014.00043
Hagström ÅK, Walther A, Wendland J, Löfstedt C (2013) Subcellular localization of the fatty acyl reductase involved in pheromone biosynthesis in the tobacco budworm, Heliothis virescens (Noctuidae: Lepidoptera). Insect Biochem Mol Biol 43:510–521. https://doi.org/10.1016/j.ibmb.2013.03.006
Heath RR, McLaughlin JR, Proshold F, Teal PEA (1991) Periodicity of female sex pheromone titer and release in Heliothis subflexa and H. virescens (Lepidoptera: Noctuidae). Ann Entomol Soc Am 84:182–189
Hellerstein MK, Neese RA (1992) Mass isotopomer distribution analysis: a technique for measuring biosynthesis and turnover of polymers. Am J Physiol Endocrinol Metab 263:E988–E1001
Johansson B, Jones T (2007) The role of chemical communication in mate choice. Biol Rev Camb Philos Soc 82:265–289. https://doi.org/10.1111/j.1469-185X.2007.00009.x
Jurenka RA (2003) Biochemistry of female moth sex pheromones. In: Blomquist GJ, Vogt RG (eds) Insect pheromone biochemistry and molecular biology. Elsevier, London, pp 53–80
Jurenka R (2017) Regulation of pheromone biosynthesis in moths. Curr Opin Insect Sci 24:29–35. https://doi.org/10.1016/j.cois.2017.09.002
Löfstedt C, Wahlberg N, Millar JG (2016) Evolutionary patterns of pheromone diversity in Lepidoptera. In: Allison JD, Cardé RT (eds) Pheromone communication in moths: evolution, behavior and application. University of California Press, Oakland, pp 43–78
Ma PWK, Ramaswamy SB (2003) Biology and ultrastructure of sex pheromone-producing tissue. In: Blomquist GJ, Vogt RC (eds) Insect pheromone biochemsitry and molecular biology. Elsevier, London, pp 19–51
Mackay D, van Wesenbeeck I (2014) Correlation of chemical evaporation rate with vapor pressure. Environ Sci Technol 48:10259–10263. https://doi.org/10.1021/es5029074
Nojima S, Classen A, Groot AT, Schal C (2018) Qualitative and quantitative analysis of chemicals emitted from the pheromone gland of individual Heliothis subflexa females. PLoS One 13:e0202035. https://doi.org/10.1371/journal.pone.0202035
Pope MM, Gaston LK, Baker TC (1982) Composition, quantification, and periodicity of sex pheromone gland volatiles from individual Heliothis virescens females. J Chem Ecol 8:1043–1055. https://doi.org/10.1007/bf00987885
Raina AK, Wergin WP, Murphy CA, Erbe EF (2000) Structural organization of the sex pheromone gland in Helicoverpa zea in relation to pheromone production and release. Arthropod Struct Dev 29:343–353
Teal PEA, Tumlinson JH (1986) Terminal steps in pheromone biosynthesis by Heliothis virescens and H. zea. J Chem Ecol 12:353–366
Turgeon JJ, McNeil JN (1983) Modifications in the calling behaviour of Pseudaletia unipuncta (Lepidoptera: Noctuidae), induced by temperature conditions during pupal and adult development. Can Entomol 115:1015–1022. https://doi.org/10.4039/Ent1151015-8
Acknowledgments
Funding for this work was provided by United States Department of Agriculture Hatch Project ND02388. We also thank the United States Department of Agriculture–National Institute of Food and Agriculture for an Instrument Grant, 2015-07238 contributing, in part, to the purchase of the GC/MS system.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foster, S.P., Anderson, K.G. & Casas, J. Calling Behavior and Sex Pheromone Release and Storage in the Moth Chloridea virescens. J Chem Ecol 46, 10–20 (2020). https://doi.org/10.1007/s10886-019-01133-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-019-01133-w